Figure 1
Team 4
Team Members |
Faculty Advisor |
Arielle Behar |
Ioulia Valla Sponsor University of Connecticut |
sponsored by
Sponsor Image Not Available
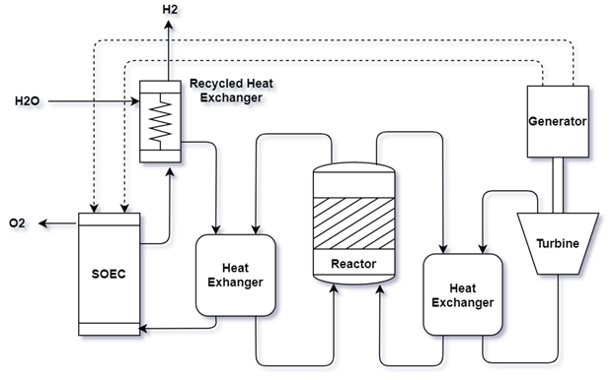
Hydrogen is a highly efficient energy carrier and has the potential to be a sustainable replacement for conventional gasoline. Currently, the primary method for commercial hydrogen production is steam methane reformation, a process which relies on unclean and unsustainable fossil fuel-derived resources. The future of hydrogen production depends on a cleaner and more reliable method using renewable resources. The goal of this project is to perform a techno-economic analysis of hydrogen production from a renewable resource for fuel cell applications. We chose nuclear-powered high-temperature electrolysis as the technology for hydrogen production. High-temperature electrolysis is a process that splits water into hydrogen and oxygen using a solid oxide electrolysis cell, and can achieve up to 90% electricity-to-H2 production efficiency with zero greenhouse gas emissions. Nuclear energy provides power and heat to the electrolysis cell to facilitate water splitting maintain a constant temperature throughout the cell. We optimized the process by investigating how temperature, voltage, and current density affect the power requirement to the cell and the hydrogen output. As the world turns more towards alternative energy sources, the demand for hydrogen production is predicted to increase and our process provides an efficient and sustainable way to produce clean hydrogen and help meet societal energy demand.
