

This video contains proprietary information and cannot be shared publicly at this time.
Figure 1

Figure 2
Team 23
Team Members |
Faculty Advisor |
Sidney Chemini |
Dr. Krystyna Gielo-Perczak Sponsor Other |
sponsored by

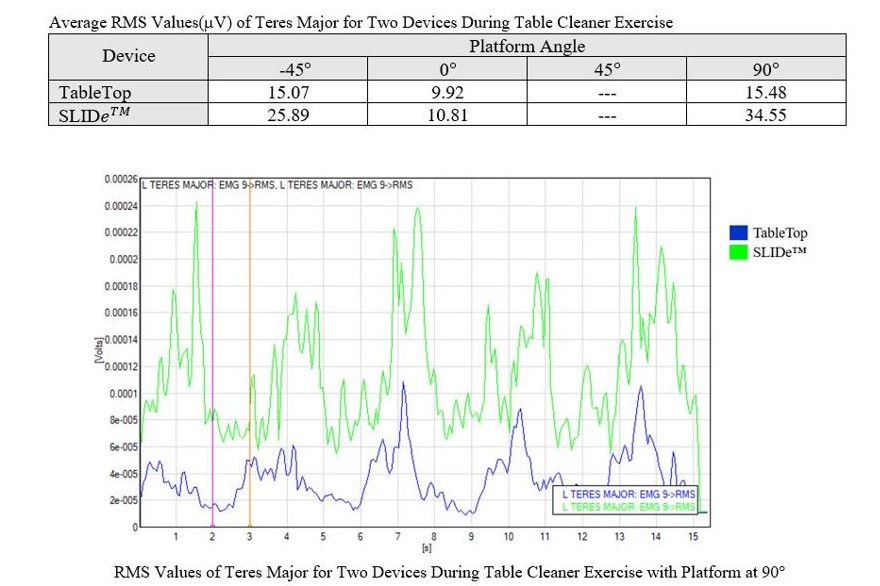
The aim of this project was two-fold: 1.) Develop and implement a biofeedback procedure within a rotator cuff rehabilitation regimen to quantify shoulder rehabilitation progression, and; 2.) Design and validate a biomechanical shoulder rehabilitation device to aid in the transition from an in-office to remote physical therapy modality due to the altering conditions created by the COVID-19 pandemic. To accomplish these aims, literary research was conducted to formulate an experimental rehabilitation procedure. To confirm that the procedure could be safely performed, human models were created using AnyBody Modeling System™ Software to simulate the desired procedure. This procedure consisted of biomechanical testing of two devices, the SLIDe™ Device and the TableTop Device. The SLIDe™ Device was originally designed by a previous senior design team and the TableTop Device was constructed as a redesigned, more compact version of the original SLIDe™ Device design. EMG sensors were placed on the Infraspinatus, Teres Major, Deltoid, Biceps Brachii, and Triceps Brachii, to obtain raw muscle activity data. This data was analyzed, and Root Mean Square (RMS) values were extrapolated to determine which device produced the optimal level of rehabilitation within the bounds of the project. This testing validated the TableTop design as a smaller and more portable version of the original design, capable of assisting in rotator cuff rehabilitation, and confirmed that this was the optimal design suited to fulfill the second aim of the project.
