
Figure 1
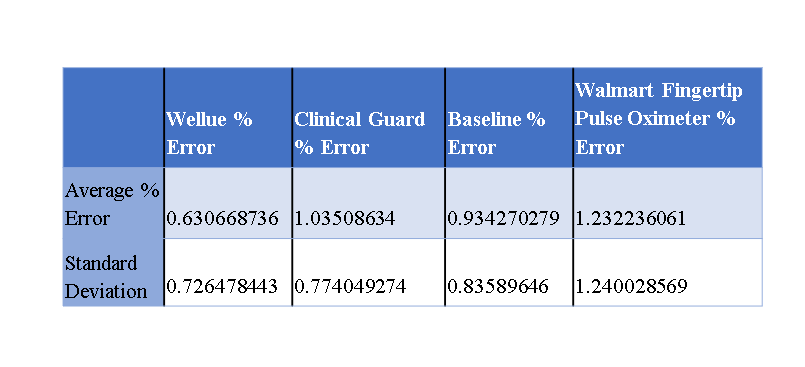
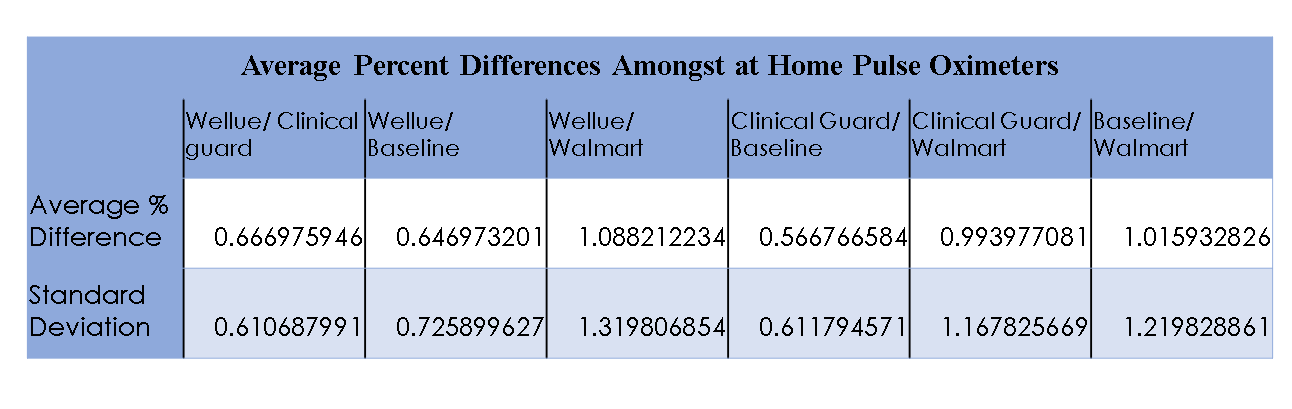
Figure 2
Team 12
Team Members |
Faculty Advisor |
Sandeepkumar Sasidharan |
Patrick Kumavor Sponsor UConn Biomedical Engineering Department |
sponsored by

The purpose of this study is to observe pulse oximeters of different price ranges and to test the accuracy between commercial oximeters and FDA-approved ones; to provide an adequate recommendation for the patient. From this information, it can influence a potential design that can be used to make a pulse oximeter that is cheaper and that is able to record information for the duration of the incubation period of COVID-19. Most pulse oximeters in the market had inaccuracies greater than the manufacturer claim (>4%). To overcome the accuracy of most pulse oximeters we plan on using a hospital-grade device as the standard for testing. We will feel comfortable giving a recommendation to a commercialized product that falls within or below 2-3% accuracy of the actual reading and if the commercial product is just as comparable to that of a hospital-grade device. This recommendation should allow for infected patients or potentially infected patients with a way to monitor the progression of the disease and identify potential severe episodes before they can occur. Allowing patients, the ability to seek proper medical care early.
