

This video contains proprietary information and cannot be shared publicly at this time.
Figure 1
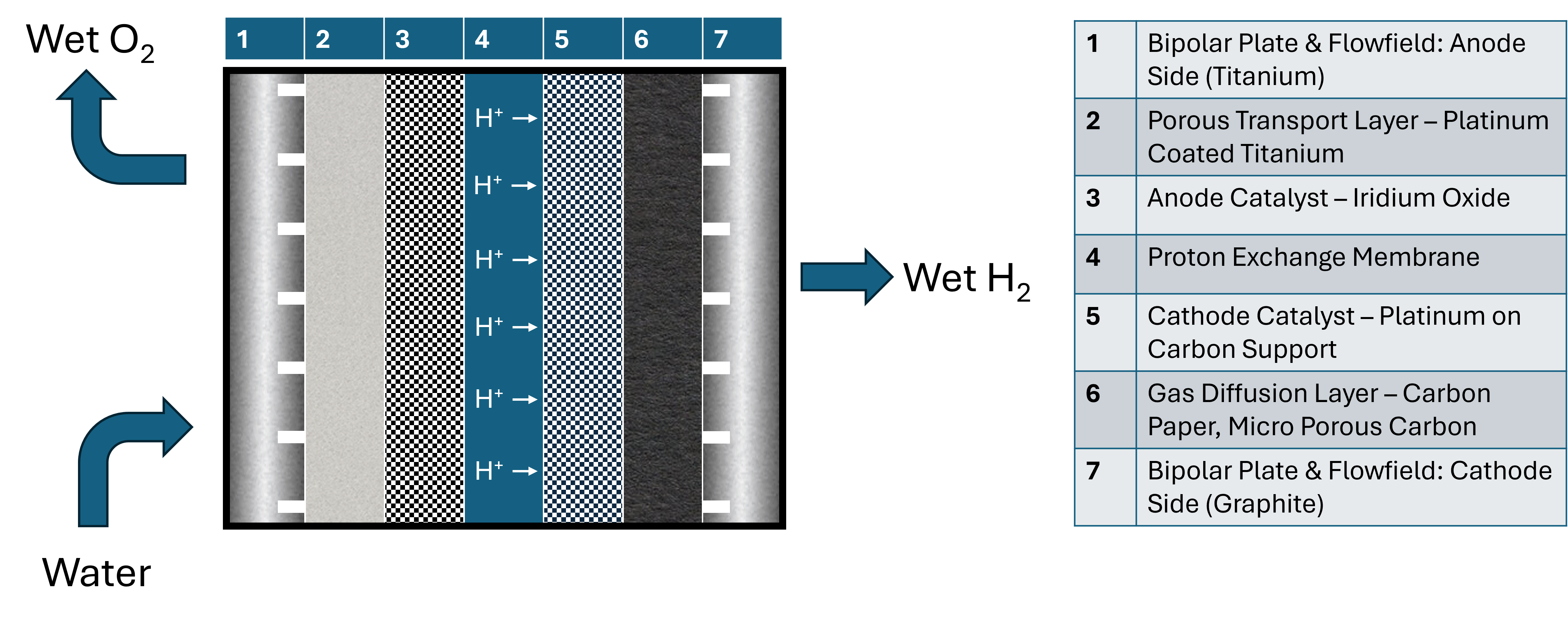
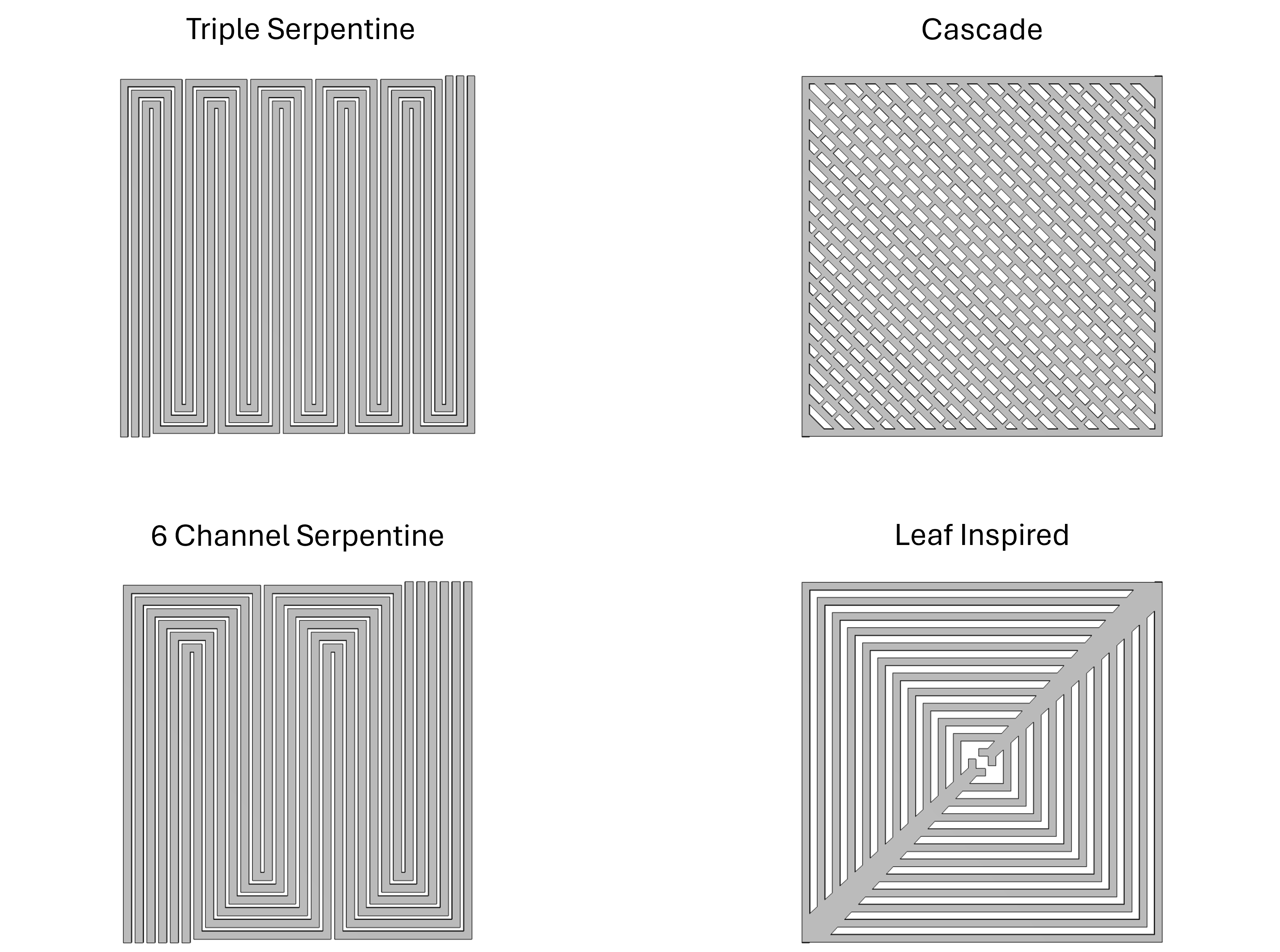
Figure 2
Team 10
Team Members |
Faculty Advisor |
Ethan Perkins |
Radenka Maric and Stoyan Bliznakov Sponsor Center for Clean Energy Engineering (C2E2) |
sponsored by

Optimizing the Cost and Performance of Proton Exchange Membrane Water Electrolysis
The current carbon economy is an unsustainable method of providing power, transportation, and resources for the modern world. The current leading alternative is the hydrogen economy. The United States Department of Energy aims to reduce the price of hydrogen to at least $1/kg by 2031 to make this path viable. An important aspect of creating a viable hydrogen economy is the process of water electrolysis. Water electrolysis is the process of splitting water molecules into their component parts of hydrogen and oxygen gas. Water electrolysers consist of backing plates, current collectors, flowfields, and a membrane electrode assembly (MEA). The MEA is made of diffusion layers, catalyst layers, and a solid electrolyte membrane. Through modification of the anode and cathode catalysts layers, the electrolyte membrane, and the flow fields, we made improvements to the water electrolysis cell. We modified anode and cathode catalyst through reactive spray deposition technology and shaped catalyst layers to reduce the amount of catalyst required for optimal performance. We improved membrane durability and performance through the addition of reinforcement and additives. Finally, we designed and tested multiple different flow field geometries to determine the best performing candidate for a cell. Through these modifications we intend to improve the performance of the cell enough to reach the 2026 Department of energy targets and get one step closer to achieving the final 2031 goals, and a sustainable hydrogen economy.
